Chapter 1. Methodology and Scope
1.1. Market Segmentation & Scope
1.2. Segment Definitions
1.2.1. Product
1.2.2. End use
1.2.3. Regional scope
1.2.4. Estimates and forecasts timeline
1.3. Research Methodology
1.4. Information Procurement
1.4.1. Purchased database
1.4.2. GVR’s internal database
1.4.3. Secondary sources
1.4.4. Primary research
1.4.5. Details of primary research
1.4.5.1. Data for primary interviews in North America
1.4.5.2. Data for primary interviews in Europe
1.4.5.3. Data for primary interviews in Asia Pacific
1.4.5.4. Data for primary interviews in Latin America
1.4.5.5. Data for Primary interviews in MEA
1.5. Information or Data Analysis
1.5.1. Data analysis models
1.6. Market Formulation & Validation
1.7. Model Details
1.7.1. Commodity flow analysis (Model 1)
1.7.2. Approach 1: Commodity flow approach
1.7.3. Volume price analysis (Model 2)
1.7.4. Approach 2: Volume price analysis
1.8. List of Secondary Sources
1.9. List of Primary Sources
1.10. Objectives
Chapter 2. Executive Summary
2.1. Market Outlook
2.2. Segment Outlook
2.2.1. Product and End use outlook
2.2.2. Regional outlook
2.3. Competitive Insights
Chapter 3. Respiratory Syncytial Virus Diagnostics Market Variables, Trends & Scope
3.1. Market Lineage Outlook
3.1.1. Parent market outlook
3.1.2. Related/ancillary market outlook
3.2. Market Dynamics
3.2.1. Market driver analysis
3.2.1.1. Rising prevalence of Respiratory Syncytial Virus (RSV) Infections
3.2.1.2. Advancements in diagnostics technologies
3.2.1.3. Growing awareness and healthcare spending
3.2.2. Market restraint analysis
3.2.2.1. High cost associated with advanced diagnostic tests
3.2.2.2. Lack of awareness in developing reagions
3.3. Respiratory Syncytial Virus Diagnostics Market Analysis Tools
3.3.1. Industry Analysis – Porter’s
3.3.1.1. Supplier power
3.3.1.2. Buyer power
3.3.1.3. Substitution threat
3.3.1.4. Threat of new entrant
3.3.1.5. Competitive rivalry
3.3.2. PESTEL Analysis
3.3.2.1. Political landscape
3.3.2.2. Economic landscape
3.3.2.3. Technological landscape
3.3.2.4. Social landscape
3.3.2.5. Environmental landscape
Chapter 4. Respiratory Syncytial Virus Diagnostics Market: Product Estimates & Trend Analysis
4.1. Global Respiratory Syncytial Virus Diagnostics Market: Product Dashboard
4.2. Global Respiratory Syncytial Virus Diagnostics Market: Product Movement Analysis
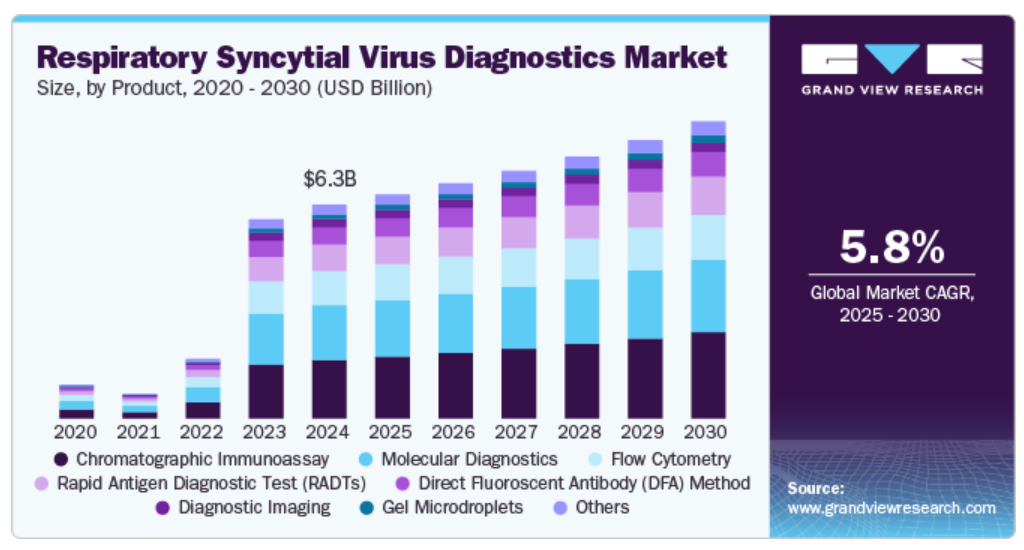
4.3. Global Respiratory Syncytial Virus Diagnostics Market by Product , Revenue
4.4. Direct Fluorescent Antibody (DFA) Method
4.5. Rapid Antigen Diagnostic Test (RADTs)
4.5.1. Rapid Antigen Diagnostic Test (RADTs) market estimates and forecasts 2018 to 2030 (USD Billion)
4.6. Molecular Diagnostics
4.6.1. Molecular diagnostics market estimates and forecasts 2018 to 2030 (USD Billion)
4.7. Chromatographic Immunoassay
4.7.1. Chromatography immunoassay market estimates and forecasts 2018 to 2030 (USD Billion)
4.7.2. Gravity Driven Test
4.7.2.1. Gravity driven test market estimates and forecasts 2018 to 2030 (USD Billion)
4.7.3. Oligochromatography (OC)
4.7.3.1. Oligochromatography (OC) market estimates and forecasts 2018 to 2030 (USD Billion)
4.8. Diagnostic Imaging
4.8.1. Diagnostic imaging market estimates and forecasts 2018 to 2030 (USD Billion)
4.9. Gel Microdroplets
4.9.1. Gel microdroplets market estimates and forecasts 2018 to 2030 (USD Billion)
4.10. Flow Cytometry
4.10.1. Flow cytometry market estimates and forecasts 2018 to 2030 (USD Billion)
4.11. Others
4.11.1. Others market estimates and forecasts 2018 to 2030 (USD Billion)
Chapter 5. Respiratory Syncytial Virus Diagnostics Market: End use Estimates & Trend Analysis
5.1. Global Respiratory Syncytial Virus Diagnostics Market: End use Dashboard
5.2. Global Respiratory Syncytial Virus Diagnostics Market: End use Movement Analysis
5.3. Global Respiratory Syncytial Virus Diagnostics Market by End use, Revenue
5.4. Hospitals
5.4.1. Hospitals market estimates and forecasts 2018 to 2030 (USD Billion)
5.5. Laboratory
5.5.1. Laboratory market estimates and forecasts 2018 to 2030 (USD Billion)
5.6. Clinics
5.6.1. Clinics market estimates and forecasts 2018 to 2030 (USD Billion)
5.7. Others
5.7.1. Others market estimates and forecasts 2018 to 2030 (USD Billion)
Chapter 6. Respiratory Syncytial Virus Diagnostics Market: Regional Estimates & Trend Analysis by Type, Tube Material, Application, and End use
6.1. Regional Dashboard
6.2. Market Size, & Forecasts Trend Analysis, 2018 to 2030:
6.3. North America
6.3.1. U.S.
6.3.1.1. Key country dynamics
6.3.1.2. Regulatory framework/ reimbursement structure
6.3.1.3. Competitive scenario
6.3.1.4. U.S. market estimates and forecasts 2018 to 2030 (USD Billion)
6.3.2. Canada
6.3.2.1. Key country dynamics
6.3.2.2. Regulatory framework/ reimbursement structure
6.3.2.3. Competitive scenario
6.3.2.4. Canada market estimates and forecasts 2018 to 2030 (USD Billion)
6.3.3. Mexico
6.3.3.1. Key country dynamics
6.3.3.2. Regulatory framework/ reimbursement structure
6.3.3.3. Competitive scenario
6.3.3.4. Mexico market estimates and forecasts 2018 to 2030 (USD Billion)
6.4. Europe
6.4.1. UK
6.4.1.1. Key country dynamics
6.4.1.2. Regulatory framework/ reimbursement structure
6.4.1.3. Competitive scenario
6.4.1.4. UK market estimates and forecasts 2018 to 2030 (USD Billion)
6.4.2. Germany
6.4.2.1. Key country dynamics
6.4.2.2. Regulatory framework/ reimbursement structure
6.4.2.3. Competitive scenario
6.4.2.4. Germany market estimates and forecasts 2018 to 2030 (USD Billion)
6.4.3. France
6.4.3.1. Key country dynamics
6.4.3.2. Regulatory framework/ reimbursement structure
6.4.3.3. Competitive scenario
6.4.3.4. France market estimates and forecasts 2018 to 2030 (USD Billion)
6.4.4. Italy
6.4.4.1. Key country dynamics
6.4.4.2. Regulatory framework/ reimbursement structure
6.4.4.3. Competitive scenario
6.4.4.4. Italy market estimates and forecasts 2018 to 2030 (USD Billion)
6.4.5. Spain
6.4.5.1. Key country dynamics
6.4.5.2. Regulatory framework/ reimbursement structure
6.4.5.3. Competitive scenario
6.4.5.4. Spain market estimates and forecasts 2018 to 2030 (USD Billion)
6.4.6. Norway
6.4.6.1. Key country dynamics
6.4.6.2. Regulatory framework/ reimbursement structure
6.4.6.3. Competitive scenario
6.4.6.4. Norway market estimates and forecasts 2018 to 2030 (USD Billion)
6.4.7. Sweden
6.4.7.1. Key country dynamics
6.4.7.2. Regulatory framework/ reimbursement structure
6.4.7.3. Competitive scenario
6.4.7.4. Sweden market estimates and forecasts 2018 to 2030 (USD Billion)
6.4.8. Denmark
6.4.8.1. Key country dynamics
6.4.8.2. Regulatory framework/ reimbursement structure
6.4.8.3. Competitive scenario
6.4.8.4. Denmark market estimates and forecasts 2018 to 2030 (USD Billion)
6.5. Asia Pacific
6.5.1. Japan
6.5.1.1. Key country dynamics
6.5.1.2. Regulatory framework/ reimbursement structure
6.5.1.3. Competitive scenario
6.5.1.4. Japan market estimates and forecasts 2018 to 2030 (USD Billion)
6.5.2. China
6.5.2.1. Key country dynamics
6.5.2.2. Regulatory framework/ reimbursement structure
6.5.2.3. Competitive scenario
6.5.2.4. China market estimates and forecasts 2018 to 2030 (USD Billion)
6.5.3. India
6.5.3.1. Key country dynamics
6.5.3.2. Regulatory framework/ reimbursement structure
6.5.3.3. Competitive scenario
6.5.3.4. India market estimates and forecasts 2018 to 2030 (USD Billion)
6.5.4. Australia
6.5.4.1. Key country dynamics
6.5.4.2. Regulatory framework/ reimbursement structure
6.5.4.3. Competitive scenario
6.5.4.4. Australia market estimates and forecasts 2018 to 2030 (USD Billion)
6.5.5. South Korea
6.5.5.1. Key country dynamics
6.5.5.2. Regulatory framework/ reimbursement structure
6.5.5.3. Competitive scenario
6.5.5.4. South Korea market estimates and forecasts 2018 to 2030 (USD Billion)
6.5.6. Thailand
6.5.6.1. Key country dynamics
6.5.6.2. Regulatory framework/ reimbursement structure
6.5.6.3. Competitive scenario
6.5.6.4. Thailand market estimates and forecasts 2018 to 2030 (USD Billion)
6.6. Latin America
6.6.1. Brazil
6.6.1.1. Key country dynamics
6.6.1.2. Regulatory framework/ reimbursement structure
6.6.1.3. Competitive scenario
6.6.1.4. Brazil market estimates and forecasts 2018 to 2030 (USD Billion)
6.6.2. Argentina
6.6.2.1. Key country dynamics
6.6.2.2. Regulatory framework/ reimbursement structure
6.6.2.3. Competitive scenario
6.6.2.4. Argentina market estimates and forecasts 2018 to 2030 (USD Billion)
6.7. MEA
6.7.1. South Africa
6.7.1.1. Key country dynamics
6.7.1.2. Regulatory framework/ reimbursement structure
6.7.1.3. Competitive scenario
6.7.1.4. South Africa market estimates and forecasts 2018 to 2030 (USD Billion)
6.7.2. Saudi Arabia
6.7.2.1. Key country dynamics
6.7.2.2. Regulatory framework/ reimbursement structure
6.7.2.3. Competitive scenario
6.7.2.4. Saudi Arabia market estimates and forecasts 2018 to 2030 (USD Billion)
6.7.3. UAE
6.7.3.1. Key country dynamics
6.7.3.2. Regulatory framework/ reimbursement structure
6.7.3.3. Competitive scenario
6.7.3.4. UAE market estimates and forecasts 2018 to 2030 (USD Billion)
6.7.4. Kuwait
6.7.4.1. Key country dynamics
6.7.4.2. Regulatory framework/ reimbursement structure
6.7.4.3. Competitive scenario
6.7.4.4. Kuwait market estimates and forecasts 2018 to 2030 (USD Billion)
Chapter 7. Competitive Landscape
7.1. Recent Developments & Impact Analysis, By Key Market Participants
7.2. Company/Competition Categorization
7.3. Vendor Landscape
7.3.1. List of key distributors and channel partners
7.3.2. Key customers
7.3.3. Key company market share analysis, 2024
7.3.4. BD (Becton, Dickinson, and Company)
7.3.4.1. Company overview
7.3.4.2. Financial performance
7.3.4.3. Product benchmarking
7.3.4.4. Strategic initiatives
7.3.5. Novartis AG
7.3.5.1. Company overview
7.3.5.2. Financial performance
7.3.5.3. Product benchmarking
7.3.5.4. Strategic initiatives
7.3.6. Abbott
7.3.6.1. Company overview
7.3.6.2. Financial performance
7.3.6.3. Product benchmarking
7.3.6.4. Strategic initiatives
7.3.7. QuidelOrtho Corporation
7.3.7.1. Company overview
7.3.7.2. Financial performance
7.3.7.3. Product benchmarking
7.3.7.4. Strategic initiatives
7.3.8. Thermo Fisher Scientific Inc.
7.3.8.1. Company overview
7.3.8.2. Financial performance
7.3.8.3. Product benchmarking
7.3.8.4. Strategic initiatives
7.3.9. Bio-Rad Laboratories, Inc.
7.3.9.1. Company overview
7.3.9.2. Financial performance
7.3.9.3. Product benchmarking
7.3.9.4. Strategic initiatives
7.3.10. F. Hoffmann-La Roche Ltd.
7.3.10.1. Company overview
7.3.10.2. Financial performance
7.3.10.3. Product benchmarking
7.3.10.4. Strategic initiatives
7.3.11. BIOMÉRIEUX
7.3.11.1. Company overview
7.3.11.2. Financial performance
7.3.11.3. Product benchmarking
7.3.11.4. Strategic initiatives
7.3.12. DiaSorin S.p.A.
7.3.12.1. Company overview
7.3.12.2. Financial performance
7.3.12.3. Product benchmarking
7.3.12.4. Strategic initiatives
7.3.13. Merck KGaA
7.3.13.1. Company overview
7.3.13.2. Financial performance
7.3.13.3. Product benchmarking
7.3.13.4. Strategic initiatives
7.3.14. Coris BioConcept
7.3.14.1. Company overview
7.3.14.2. Financial performance
7.3.14.3. Product benchmarking
7.3.14.4. Strategic initiatives
7.3.15. Siemens Healthineers AG
7.3.15.1. Company overview
7.3.15.2. Financial performance
7.3.15.3. Product benchmarking
7.3.15.4. Strategic initiatives
7.3.16. Quest Diagnostics Incorporated
7.3.16.1. Company overview
7.3.16.2. Financial performance
7.3.16.3. Product benchmarking
7.3.16.4. Strategic initiatives
List of Tables
Table 1 List of Secondary Sources
Table 2 List of Abbreviations
Table 3 Global Oncology Companion Diagnostic, by Product, 2018 – 2030 (USD Billion)
Table 4 Global Oncology Companion Diagnostic, by End use, 2018 – 2030 (USD Billion)
Table 5 Global Oncology Companion Diagnostic, by Region, 2018 – 2030 (USD Billion)
Table 6 North America Oncology Companion Diagnostic, by Country, 2018 – 2030 (USD Billion)
Table 7 North America Oncology Companion Diagnostic, by Product, 2018 – 2030 (USD Billion)
Table 8 North America Oncology Companion Diagnostic, by End use, 2018 – 2030 (USD Billion)
Table 9 U.S. Oncology Companion Diagnostic, by Product, 2018 – 2030 (USD Billion)
Table 10 U.S. Oncology Companion Diagnostic, by End use, 2018 – 2030 (USD Billion)
Table 11 Canada Oncology Companion Diagnostic, by Product, 2018 – 2030 (USD Billion)
Table 12 Canada Oncology Companion Diagnostic, by End use, 2018 – 2030 (USD Billion)
Table 13 Mexico Oncology Companion Diagnostic, by Product, 2018 – 2030 (USD Billion)
Table 14 Mexico Oncology Companion Diagnostic, by End use, 2018 – 2030 (USD Billion)
Table 15 Europe Oncology Companion Diagnostic, by Country, 2018 – 2030 (USD Billion)
Table 16 Europe Oncology Companion Diagnostic, by Product, 2018 – 2030 (USD Billion)
Table 17 Europe Oncology Companion Diagnostic, by End use, 2018 – 2030 (USD Billion)
Table 18 Germany Oncology Companion Diagnostic, by Product, 2018 – 2030 (USD Billion)
Table 19 Germany Oncology Companion Diagnostic, by End use, 2018 – 2030 (USD Billion)
Table 20 UK Oncology Companion Diagnostic, by Product, 2018 – 2030 (USD Billion)
Table 21 UK Oncology Companion Diagnostic, by End use, 2018 – 2030 (USD Billion)
Table 22 France Oncology Companion Diagnostic, by Product, 2018 – 2030 (USD Billion)
Table 23 France Oncology Companion Diagnostic, by End use, 2018 – 2030 (USD Billion)
Table 24 Italy Oncology Companion Diagnostic, by Product, 2018 – 2030 (USD Billion)
Table 25 Italy Oncology Companion Diagnostic, by End use, 2018 – 2030 (USD Billion)
Table 26 Spain Oncology Companion Diagnostic, by Product, 2018 – 2030 (USD Billion)
Table 27 Spain Oncology Companion Diagnostic, by End use, 2018 – 2030 (USD Billion)
Table 28 Denmark Oncology Companion Diagnostic, by Product, 2018 – 2030 (USD Billion)
Table 29 Denmark Oncology Companion Diagnostic, by End use, 2018 – 2030 (USD Billion)
Table 30 Sweden Oncology Companion Diagnostic, by Product, 2018 – 2030 (USD Billion)
Table 31 Sweden Oncology Companion Diagnostic, by End use, 2018 – 2030 (USD Billion)
Table 32 Norway Oncology Companion Diagnostic, by Product, 2018 – 2030 (USD Billion)
Table 33 Norway Oncology Companion Diagnostic, by End use, 2018 – 2030 (USD Billion)
Table 34 Asia Pacific Oncology Companion Diagnostic, by Country, 2018 – 2030 (USD Billion)
Table 35 Asia Pacific Oncology Companion Diagnostic, by Product, 2018 – 2030 (USD Billion)
Table 36 Asia Pacific Oncology Companion Diagnostic, by End use, 2018 – 2030 (USD Billion)
Table 37 China Oncology Companion Diagnostic, by Product, 2018 – 2030 (USD Billion)
Table 38 China Oncology Companion Diagnostic, by End use, 2018 – 2030 (USD Billion)
Table 39 Japan Oncology Companion Diagnostic, by Product, 2018 – 2030 (USD Billion)
Table 40 Japan Oncology Companion Diagnostic, by End use, 2018 – 2030 (USD Billion)
Table 41 India Oncology Companion Diagnostic, by Product, 2018 – 2030 (USD Billion)
Table 42 India Oncology Companion Diagnostic, by End use, 2018 – 2030 (USD Billion)
Table 43 South Korea Oncology Companion Diagnostic, by Product, 2018 – 2030 (USD Billion)
Table 44 South Korea Oncology Companion Diagnostic, by End use, 2018 – 2030 (USD Billion)
Table 45 Australia Oncology Companion Diagnostic, by Product, 2018 – 2030 (USD Billion)
Table 46 Australia Oncology Companion Diagnostic, by End use, 2018 – 2030 (USD Billion)
Table 47 Thailand Oncology Companion Diagnostic, by Product, 2018 – 2030 (USD Billion)
Table 48 Thailand Oncology Companion Diagnostic, by End use, 2018 – 2030 (USD Billion)
Table 49 Latin America Oncology Companion Diagnostic, by Country, 2018 – 2030 (USD Billion)
Table 50 Latin America Oncology Companion Diagnostic, by Product, 2018 – 2030 (USD Billion)
Table 51 Latin America Oncology Companion Diagnostic, by End use, 2018 – 2030 (USD Billion)
Table 52 Brazil Oncology Companion Diagnostic, by Product, 2018 – 2030 (USD Billion)
Table 53 Brazil Oncology Companion Diagnostic, by End use, 2018 – 2030 (USD Billion)
Table 54 Argentina Oncology Companion Diagnostic, by Product, 2018 – 2030 (USD Billion)
Table 55 Argentina Oncology Companion Diagnostic, by End use, 2018 – 2030 (USD Billion)
Table 56 Middle East & Africa Oncology Companion Diagnostic, by Country, 2018 – 2030 (USD Billion)
Table 57 Middle East & Africa Oncology Companion Diagnostic, by Product, 2018 – 2030 (USD Billion)
Table 58 Middle East & Africa Oncology Companion Diagnostic, by End use, 2018 – 2030 (USD Billion)
Table 59 South Africa Oncology Companion Diagnostic, by Product, 2018 – 2030 (USD Billion)
Table 60 South Africa Oncology Companion Diagnostic, by End use, 2018 – 2030 (USD Billion)
Table 61 Saudi Arabia Oncology Companion Diagnostic, by Product, 2018 – 2030 (USD Billion)
Table 62 Saudi Arabia Oncology Companion Diagnostic, by End use, 2018 – 2030 (USD Billion)
Table 63 UAE Oncology Companion Diagnostic, by Product, 2018 – 2030 (USD Billion)
Table 64 UAE Oncology Companion Diagnostic, by End use, 2018 – 2030 (USD Billion)
Table 65 Kuwait Oncology Companion Diagnostic, by Product, 2018 – 2030 (USD Billion)
Table 66 Kuwait Oncology Companion Diagnostic, by End use, 2018 – 2030 (USD Billion)